The South African Health Products Regulatory Authority (SAHPRA) on Monday said it had registered two more Covid-19 jabs – the COMIRNATY vaccine by Pfizer Laboratories (Pty) Ltd and the Covid-19 VACCINE MC PHARMA by MC Pharma (Pty) Ltd.
At the weekend, The Bulrushes reported that the SAHPPRA would announce Monday the names of the latest Covid-19 vaccination to be authorised for use in South Africa. For more read: REGISTERED: SAHPRA has auhtorised the use of these two Covid-19 jabs
Sources had correctly told The Bulrushes that at least one of the vaccines was from China.
“The registration of these vaccines is a vast stride in vaccine registration as SAHPRA plays its role in the fight against Covid-19,” said SAHPRA CEO Dr. Boitumelo Semete-Makokotlela.
“SAHPRA will continue to play its part in ensuring the quality, safety, and efficacy of all health products, including all vaccines to ensure that the South African public is protected at all times.”
COMIRNATY is an mRNA vaccine, indicated for active immunisation to prevent Covid-19 in individuals 12 years of age and older. It is administered intramuscularly after dilution as a course of 2 doses (0,3 mL each).
SAHPRA said it was recommended that the second dose be administered three weeks after the initial dose.
“This authorisation is based on acceptable safety, quality and efficacy data submitted by Pfizer Laboratories (Pty) Ltd to SAHPRA as a rolling submission over the period 3 February 2021 to 17 January 2022,” explained the statement.
“The authorisation is, however, subject to a number of conditions which includes that the vaccine is supplied and administered in accordance with the National Covid-19 vaccination programme and applicable guidelines.
“Further conditions relate to the reporting of the results of ongoing studies and conformance with pharmacovigilance activities as outlined in the approved risk management plan, including the submission of periodic safety updates.”
SAHPRA said the adverse effects of the COMIRNATY vaccine, as outlined in the clinical trial evidence submitted by the applicant, were usually mild or moderate and cleared within a few days of vaccination.
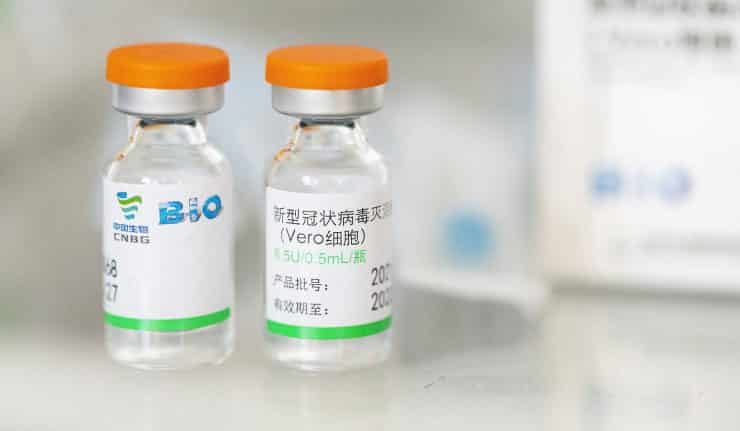
The Covid-19 VACCINE MC PHARMA is an inactivated Vero Cell vaccine, indicated for immunisation against SARS-CoV-2 in those aged 18 years and older.
Sources had correctly told The Bulrushes that at least one of the vaccines was from China.
Initially developed by the Beijing Institute of Biological Products Co., Ltd, this product has also been referred to as the Sinopharm/BIBP vaccine indicated for immunisation against SARS-CoV-2.
The Covid-19 VACCINE MC PHARMA is administered in two doses by intramuscular injection at an interval of 2-4 weeks and each dose is 0.5ml.
“This authorisation is based on acceptable safety, quality, and efficacy data submitted by MC Pharma Pty (Ltd) to SAHPRA as a rolling submission over the period 23 July 2021 to 22 December 2021,” said the statement.
“The authorisation is, however, subject to a number of conditions which includes that the vaccine is supplied and administered in accordance with the National Covid-19 vaccination programme.
“Further conditions relate to the reporting of the results of ongoing studies and conformance with pharmacovigilance activities as outlined in the approved risk management plan, including the submission of periodic safety updates.”
SAHPRA said adverse effects of the Covid-19 VACCINE MC PHARMA, as outlined in the clinical trial evidence submitted by the applicant, were usually mild or moderate and cleared within a few days of vaccination.